Post a message
Replying to:
Langerhans cell
Described by Paul Langerhans in 1868 (#Langerhans, 1868) , Langerhans cells which represent 3-6% of all cells in the epidermis , are mobile, dendritic, antigen presenting cells with a stellate appearance which reside in the suprabasal layers of the epidermis wedged in between, and in close contact with keratinocytes.
1. Morphology of Langerhans cells
Ultrastructurally, LCs have an indented or lobulated nucleus and an electron-lucent cytoplasm, devoid of tonofilaments and melanosomes. They were initially identified by a specific cytoplasmic marker , the Birbeck granule , an electron-dense organelle which has a unique tennis-racket appearance (#Birbeck et al., 1961). The function of Birbeck granules is still unclear, but likely includes receptor-mediated endocytosis and transport of cellular materials into the extracellular space.

- Transmission electron microscopy view of a Langerhans cell in this epidermis of a human skin, two months after transplantation onto the nude mouse (X 10000).
- Note the Birbeck granules and the absence of desmosomes with the neighbouring keratinocytes.

- High magnification of a Birbeck granule (GB) viewed with a transmission electron microscope (X 100 000)
By immunohistochemical techniques, LCs may be identified by using a monoclonal antibody to CD1a, an MHC-I-like molecule that presents microbial lipids to T cells and more recently, by the monoclonal antibody to Langerin/CD207, a membranous C-type lectin that recognizes mannosylated ligands found on the surface of a wide range of pathogens including viruses, bacteria, fungi, and protozoa (#Valladeau et al., 2000). Following antigen activation and receptor mediated endocytosis, CD1a and Langerin/CD207 traffic to the Birbeck granule where they may participate in antigen processing.
2. Where do Langerhans cells come from?
2.1 Human Langerhans cell origin
Earlier studies showed that after allogeneic bone marrow transplants, LCs are completely replaced by donor cells within a few weeks; this led to the concept that LCs are derived from a mobile pool of bone marrow -derived precursors that are constantly recruited to the skin (#Katz et al., 1979).
Later, experiments made with the model of the human skin grafted onto the nude mouse shed new light on the origin and trafficking of human LCs during steady-state . In this system, the donor human LCs persist for the life of the graft despite epidermal turnover and absence of circulating human precursors. It was also shown that when a wound is created at the center of a normal human skin graft, human LCs originating from the surrounding non-injured human epidermis repopulate the wounded epidermis (#Démarchez et al., 1993).
To investigate whether the mouse LCs may repopulate human skin devoid of human LCs, two different systems of human skin equivalent have been grafted onto the nude mouse (#Démarchez et al., 1993). They were composed of human keratinocytes deposited on dead human dermis, or on lattice composed of human fibroblasts embedded in type I collagen. As expected, human LCs were absent in both systems before and after grafting. By indirect immunofluorescence and electron microscopy , mouse LCs were observed to repopulate the human epidermis of both types of human skin equivalents. In fact, it appeared that, at long periods (5 and 12 months) after transplantation of human skin onto the nude mouse, both murine and human LCs were present in the human epidermis.
2.2 Self -reproducing potential of Langerhans cell in human skin
By flow cytometric studies performed with epidermal cell suspensions issued from human skin, Czernielewski et al. had already shown that human LCs may be found in different phases of the cell cycle. In order to answer the question as whether human epidermal LCs may actively cycle in the epidermis, (#Czernielewski and Démarchez, 1987) have used bromodeoxyuridine (BrdU), an analogue that incorporates specifically into DNA during the S phase of the cell cycle to estimate the human LC replicating potential in human skin grafted onto nude mice. Mice bearing human grafts were injected s.c. every 6h for up to 17 days with BrdU. Human LCs that had incorporated BrdU were revealed by immunofluorescent double labeling with antibodies specific to BrdU or human LCs. It was observed that the number of BrdU-positive human LCs increased in a linear manner (4.9%, 34%, 67%, and 94% respectively at 6h, 120h, 240, and 400 h), during the course of continuous labeling procedures. Based on this result, a total cell cycle time of 392h (16.3 days) and of 12h for the S-phase was calculated for human epidermal LCs.
Applying this technique, it was also shown that after local treatment with 12-O-tetradecanoylphorbol-13-acetate or after tape stripping, the number of BrdU-labeled LCs considerably increased. Furthermore, after injection of colchicine, a metaphase blocker in the nude mice, human LCs undergoing mitosis were evidenced by electron microscopy in the grafted human epidermis.
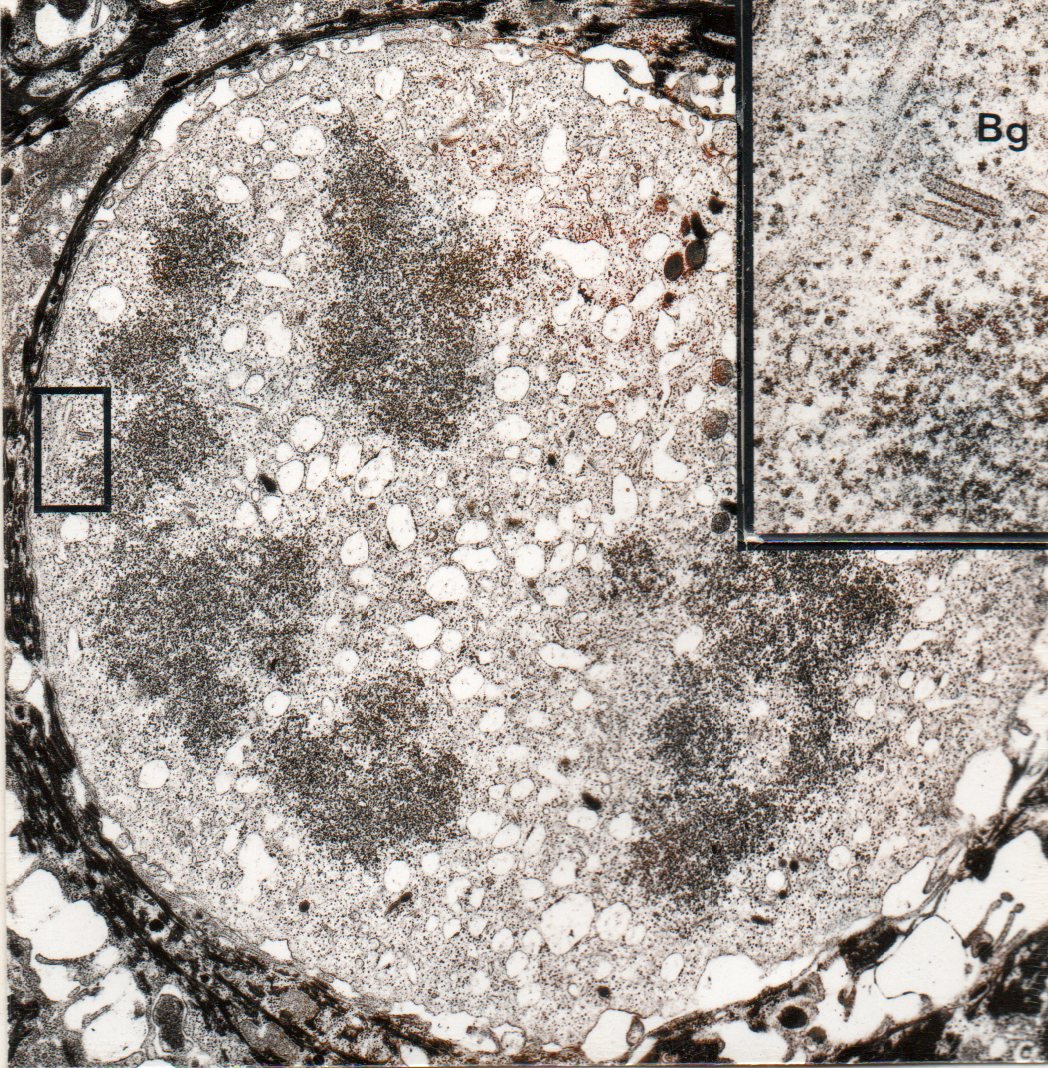
- Electron microscopy picture of Langerhans cell undergoing mitosis
- Inset : detail showing Birbeck granules (Bg)
From these data, it appears that LCs are all actively cycling (passing through the S-phase and cell division) and constitute, in normal epidermis, a stable self-reproducing cell population throughout life under steady-state conditions. However, these conclusions are not incompatible with the fact that circulating LC precursors are recruited in addition, to the skin under conditions that resulted in an exaggerated loss of resident LCs.
2.3 Data with mouse skin
More recently, elegant studies from Merad et al. confirmed in mouse skin these observations made with human skin. They observed that circulating LC precursors fail to enter noninflamed skin in two independent experimental models (#Merad et al., 2002). First, for at least 18 months after congenic bone marrow transplantation, the majority of LCs were still of host origin, whereas nearly all circulating leukocytes, including monocytes and DCs, were of donor origin by 8 weeks after transplantation. Second, there was no mixing of LCs in congenic parabiotic mice with separate organs but that shared a single blood circulation. However, in skin exposed to ultraviolet light, LCs rapidly disappeared and were replaced by circulating LC precursors within 2 weeks. The recruitment of new LCs was dependent on their expression of the CCR2 chemokine receptor and on the secretion of CCR2-binding chemokines by inflamed skin. In additional studies, when newborn or adult mice were transplanted with congenic fetal liver or bone marrow cells, no LC chimerism was detectable in the skin for more than 6 months, despite rapid chimerism in bone marrow, spleen and lymph nodes . This suggested that as soon as 1 day after birth, LCs become autonomous in the skin. This was consistent with reports of the presence of differentiated LCs in the epidermis of fetal mice, rats and humans. These data indicate that under steady-state conditions, LCs are maintained locally, but inflammatory changes in the skin result in their replacement by blood-borne LC progenitors.
3. Functions of Langerhans Cells
Presently, there is much debate concerning the physiological role of LCs.
Until recently it was assumed that LCs were antigen presenting cells that locally process cutaneous antigens and then migrate out of the skin into the draining lymph node for efficient antigen presentation to T cells. During this journey to the lymph node, the LCs change their surface phenotype and “mature”, simultaneously down-regulating antigen-processing and acquiring improved ability for T cell co-stimulation (#Stoitzner et al., 2002).
However, recent studies have questioned the biological significance of this pathway as antigen-specific T cell activation remains intact in LC deficient murine models. However, inconsistent results were obtained in these studies, which may be due to many factors, such as the mode of LC depletion (constitutive or induced), the site of challenge (epidermis versus epidermis and dermis), the dose of antigen, and the timing of contact hypersensitivity schedules. To date, no model claiming to show the redundancy of LCs in the induction of immunity has used a strictly epidermotropic pathogen, e.g papillomavirus.
A novel role for LCs was recently suggested since it was shown that LCs expressing ligands for cytotoxic T cells unexpectedly promoted murine carcinogenesis. These new observations suggest that there is steady-state migration of LCs to skin-draining lymph nodes, perhaps to induce and maintain tolerance to cutaneous antigens. Thus a working hypothesis is emerging whereby DCs that reside in the dermis may be essential for the process of cutaneous immune activation while LCs may play a more important role in sustaining cutaneous immunological tolerance. It could also be that LCs has the ability to play one or the other of this two roles depending of the environmental context.
Bibliography
Birbeck MS, Breathnach AS, Everall JD. An electron microscope study of basal melanocytes and high-level clear cells (Langerhans cells) in vitiligo . J Invest Dermatol 1961 ; 37: 51-64.
Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature 1992 ; 360 : 258-61.
Czernielewski JM, Démarchez M. Further evidence for the self-reproducing capacity of Langerhans cells in human skin. J Invest Dermatol. 1987 Jan;88(1):17-20. (link)
Démarchez M, Asselineau D, Czernielewski J. Migration of Langerhans cells into human epidermis of “reconstructed” skin, normal skin, or healing skin, after grafting onto the nude mouse.
J Invest Dermatol. 1993 May;100(5):648-52. [link]
Geissmann F, Prost C, Monnet JP, et al. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med 1998 ; 187 : 961-6.
Hacker C, Kirsch RD, Ju XS, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol 2003 ; 4 : 380-6.
Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature 1979 ; 282: 324-6.
Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 2005 ; 22 : 643-54.
Langerhans P. Uber die Nerven der menschlichen Haut. Virchows Arch Path Anat 1868 ; 44 : 325-37.
Merad M, Manz MG, Karsunky H, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol 2002 ; 3 : 1135-41. (Link)
Stoitzner P, Pfaller K, Stossel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol 2002 ; 118 : 117-25.[link]
Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 2000 ; 12 : 71-81.
Site powered by SPIP 3.0.17 + AHUNTSIC
Visitors logged in: 61
